STEMart, a US-based provider of comprehensive services for all phases of medical device development, recently announced the launch of new Radiation Quarterly Dose Audits (QDAs) Services in accordance with the Radiation Sterilization Standard ANSI/AAMI/ISO 11137 and technical specification ANSI/AAMI/ISO 13004.
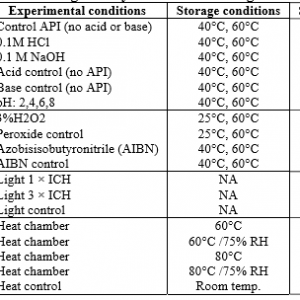
Quarterly Dose Audits are used to monitor the frequency of audits (quarterly, with the possibility of less frequent audits), validation doses, and argumentative doses in the event of a failed product audit. Dose audits are an effective program for controlling microbial levels in the manufacturing environment and are critical to minimizing the bioburden of the medical devices produced.
ISO 11137, "Sterilization of health care products - requirements for validation and routine control - radiation sterilization" provides certain criteria that should be met prior to the production of a medical device and outlines the general procedures for auditing the sterilization dose to ensure that the dose is validated in the event of a change in the production process.
Radiation Sterilization Validation is the dose of radiation required to reduce the cellular population of the indicator microorganism by 90% and determines the appropriate radiation sterilization dose for products requiring a sterility claim. It depends on many factors, including temperature, humidity, organism species, chemical environment, or the physical surface to which the indicator microorganism is attached. Bacillus Pumilus spores are the U.S. Pharmacopeia (USP) bioindicator of choice for radiation sterilization. This includes bioburden testing, bioburden recovery efficiency testing, radiation dose verification, sterility testing, and bacteriostatic or fungal testing.
Bioburden testing determines the number of viable microorganisms on or in a product. This test is performed prior to sterilization but after all other production steps, including packaging. The key to this test is a recovery efficiency test to determine the effectiveness of the bioburden extraction method in removing microorganisms from the product.
Sterility testing is performed on irradiated products for sterility. The key to sterility testing is to perform a bacteriostatic/fungal test to demonstrate the absence of bacteriostatic action in the sterility test system, which is necessary to validate the sterility test. The bioburden results are compared to the tables in the standard to determine the appropriate validation dose. The validation dose is then applied to the desired amount of product.
STEMart's QDA service is performed by a team of experienced professionals using state-of-the-art radiation dose measurement equipment. The results of the audit are then reported back to the manufacturer. The QDA involves only a single batch of samples and involves the use of the validation dose determined during validation, not the setting of a new dose.
If you have any questions about Radiation Quarterly Dose Audits Services or would like to learn more about medical device testing services, please visit https://www.ste-mart.com/radiation-quarterly-dose-audits-qdas.htm.
About STEMart
STEMart is an industry-leading eCommerce platform incorporated with an extensive global footprint and a broad portfolio of more than 10,000 products. It aims to provide better lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage upward to the manufacturing process. STEMart is dedicated to enhancing research and biotech production with simpler and safer protocols in order to access better health worldwide.
Quarterly Dose Audits are used to monitor the frequency of audits (quarterly, with the possibility of less frequent audits), validation doses, and argumentative doses in the event of a failed product audit. Dose audits are an effective program for controlling microbial levels in the manufacturing environment and are critical to minimizing the bioburden of the medical devices produced.
ISO 11137, "Sterilization of health care products - requirements for validation and routine control - radiation sterilization" provides certain criteria that should be met prior to the production of a medical device and outlines the general procedures for auditing the sterilization dose to ensure that the dose is validated in the event of a change in the production process.
Radiation Sterilization Validation is the dose of radiation required to reduce the cellular population of the indicator microorganism by 90% and determines the appropriate radiation sterilization dose for products requiring a sterility claim. It depends on many factors, including temperature, humidity, organism species, chemical environment, or the physical surface to which the indicator microorganism is attached. Bacillus Pumilus spores are the U.S. Pharmacopeia (USP) bioindicator of choice for radiation sterilization. This includes bioburden testing, bioburden recovery efficiency testing, radiation dose verification, sterility testing, and bacteriostatic or fungal testing.
Bioburden testing determines the number of viable microorganisms on or in a product. This test is performed prior to sterilization but after all other production steps, including packaging. The key to this test is a recovery efficiency test to determine the effectiveness of the bioburden extraction method in removing microorganisms from the product.
Sterility testing is performed on irradiated products for sterility. The key to sterility testing is to perform a bacteriostatic/fungal test to demonstrate the absence of bacteriostatic action in the sterility test system, which is necessary to validate the sterility test. The bioburden results are compared to the tables in the standard to determine the appropriate validation dose. The validation dose is then applied to the desired amount of product.
STEMart's QDA service is performed by a team of experienced professionals using state-of-the-art radiation dose measurement equipment. The results of the audit are then reported back to the manufacturer. The QDA involves only a single batch of samples and involves the use of the validation dose determined during validation, not the setting of a new dose.
If you have any questions about Radiation Quarterly Dose Audits Services or would like to learn more about medical device testing services, please visit https://www.ste-mart.com/radiation-quarterly-dose-audits-qdas.htm.
About STEMart
STEMart is an industry-leading eCommerce platform incorporated with an extensive global footprint and a broad portfolio of more than 10,000 products. It aims to provide better lab materials, medical instruments and consumables, excellent technologies, and high-quality services to global customers in the fields of science, technology, and engineering, from the discovery stage upward to the manufacturing process. STEMart is dedicated to enhancing research and biotech production with simpler and safer protocols in order to access better health worldwide.