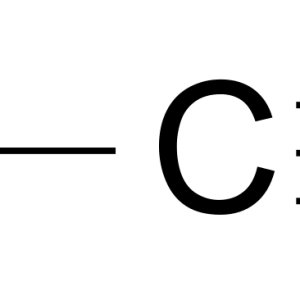The heavy dependence on petroleum-derived fuel has raised concerns about energy sustainability and climate change, which have prompted researchers to explore fuel production from renewable sources. 1-Butanol and isobutanol are promising biofuels that have favorable properties and can also serve as solvents or chemical feedstocks. Microbial production of these alcohols provides great opportunities to access a wide spectrum of renewable resources. In recent years, research has improved the native 1-butanol boiling point production and has engineered isobutanol production in various organisms to explore metabolic diversity and a broad range of substrates. This review focuses on progress in metabolic engineering for the production of these two compounds using various resources.
About a century ago, 1-butanol was first commercially produced by solventogenic Clostridium acetobutylicum using starch or sugar. The so-called ABE fermentation co-produced acetone, butanol and ethanol during the fermentation process. In spite of its early success, the bio-based production of 1-butanol boiling point was outcompeted by the petroleum-based processes after 1950s. However, bioproduction of butanol has regained interest owing to the need for deriving fuels and chemicals from renewable resources. As such, intensive effort has been made to improve ABE fermentation in various aspects including utilization of low-cost feedstock, solvent (butanol) tolerance, oxygen tolerance, product selectivity and improving cell density and sustainable viability. On the other hand, the butanol-production pathway has been introduced to various host organisms with an aim to explore a wider range of metabolic capability. For instance, the pathway was expressed in Escherichia coli and Saccharomyces cerevisiae for their high growth rates and the efficiency of genetic tools. Pseudomonas putida, Lactobacillus brevis and Bacillus subtilis were used for their potentially higher solvent tolerance. Cyanobacterium Synechococcus elongatus was used for direct conversion of CO2 to butanol. Details of 1-butanol boiling point in various organisms are summarized in Table 1. These approaches opened new opportunities in reviving bio-based production of 1-butanol. However, except an engineered E. coli strain that successfully produced 15 g L−1 of 1-butanol, comparable to the native Clostridium producers, all other organisms only produced less than 1 g L−1 of 1-butanol, leaving significant room for further work.
Similar to 1-butanol, isobutanol is an attractive option for biofuel. Its energy density is similar to that of 1-butanol but possesses a higher octane number, which is preferable for blending into gasoline. Biological isobutanol production with a significant titer and yield was first demonstrated in E. coli using a non-fermentative pathway. The high yield production suggested its potential for commercialization. The production strategy has been extended to a wide range of organisms including yeast, a cyanobacterium, an anaerobic, thermophilic and cellulolytic organism and a lithoautotroph that can utilize inorganic compounds as an energy source to fix carbon dioxide.
This review focuses on metabolic engineering of 1-butanol and isobutanol productions using various organisms. As the feedstock for butanol bioproduction is a critical factor for its economic feasibility, utilization of different resources for production is also discussed.
About a century ago, 1-butanol was first commercially produced by solventogenic Clostridium acetobutylicum using starch or sugar. The so-called ABE fermentation co-produced acetone, butanol and ethanol during the fermentation process. In spite of its early success, the bio-based production of 1-butanol boiling point was outcompeted by the petroleum-based processes after 1950s. However, bioproduction of butanol has regained interest owing to the need for deriving fuels and chemicals from renewable resources. As such, intensive effort has been made to improve ABE fermentation in various aspects including utilization of low-cost feedstock, solvent (butanol) tolerance, oxygen tolerance, product selectivity and improving cell density and sustainable viability. On the other hand, the butanol-production pathway has been introduced to various host organisms with an aim to explore a wider range of metabolic capability. For instance, the pathway was expressed in Escherichia coli and Saccharomyces cerevisiae for their high growth rates and the efficiency of genetic tools. Pseudomonas putida, Lactobacillus brevis and Bacillus subtilis were used for their potentially higher solvent tolerance. Cyanobacterium Synechococcus elongatus was used for direct conversion of CO2 to butanol. Details of 1-butanol boiling point in various organisms are summarized in Table 1. These approaches opened new opportunities in reviving bio-based production of 1-butanol. However, except an engineered E. coli strain that successfully produced 15 g L−1 of 1-butanol, comparable to the native Clostridium producers, all other organisms only produced less than 1 g L−1 of 1-butanol, leaving significant room for further work.
Similar to 1-butanol, isobutanol is an attractive option for biofuel. Its energy density is similar to that of 1-butanol but possesses a higher octane number, which is preferable for blending into gasoline. Biological isobutanol production with a significant titer and yield was first demonstrated in E. coli using a non-fermentative pathway. The high yield production suggested its potential for commercialization. The production strategy has been extended to a wide range of organisms including yeast, a cyanobacterium, an anaerobic, thermophilic and cellulolytic organism and a lithoautotroph that can utilize inorganic compounds as an energy source to fix carbon dioxide.
This review focuses on metabolic engineering of 1-butanol and isobutanol productions using various organisms. As the feedstock for butanol bioproduction is a critical factor for its economic feasibility, utilization of different resources for production is also discussed.