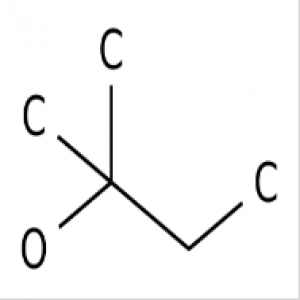
Here’s the thing: Chromic acid, H2CrO4, is a strong acid and a reagent for oxidizing alcohols to ketones and carboxylic acids. For fairly mundane reasons owing primarily to safety and convenience, chromic acid tends to be made in the reaction vessel as needed (through addition of acid to a source of chromium), rather than being dispensed from a bottle.
And that’s where the trouble begins. Choosing a source of chromium to make H2CrO4 from is a lot like choosing a favorite brand of bottled water. Beyond the packaging, they’re pretty much all the same. Depending on which textbook or instructor you have, however, you might see several different ways to do this, and it can be very confusing.
The key point is that Na2CrO4 (sodium chromate), Na2Cr2O7 (sodium dichromate), K2CrO4 (potassium chromate), K2Cr2O7 (potassium dichromate), and CrO3 (chromium trioxide) are all alike in one crucial manner: when they are combined with aqueous acid, each of them forms H2CrO4, and ultimately it’s H2CrO4 which does the important chemistry. Unfortunately I rarely see this point explained in textbooks. I remember this causing some confusion for me when I took the course. The K or Na ions present are just spectators.
H2CrO4 As A Reagent For The Oxidation Of Alcohols
Once H2CrO4 is formed, its reactions are pretty straightforward: it converts primary alcohols (and aldehydes) to carboxylic acids and secondary alcohols to ketones.
I wish I could tell you that navigating this confusion is ultimately rewarding due to the vast usefulness of H2CrO4 as a reagent. In fact, due to its high toxicity, chromic acid tends to find very little use in the organic chemistry laboratory outside of undergrad labs. There are far more useful reagents out there for performing these transformations.
Molecular chromic acid – H2CrO4 is similar to sulphuric acid (H2SO4) as both are strong acids, however, only the first proton is lost easily.
Dichromic acid – H2Cr2O7 is the fully protonated form of the dichromate (Cr2O7–) ion. Also, it is seen as the product of adding chromium trioxide (CrO3) to molecular chromic acid.
Uses of Chromic acid (H2CrO4)
Chromic acid acts as an intermediate in chromium plating.
It is used in ceramic glazes and coloured glass.
Chromosulfuric acid or sulfochromic mixture is a strong oxidizing agent that is used to clean laboratory glassware.
It has the ability to brighten raw brass and therefore it is used in the instrument repair industry.
In the year 1940, it was used in hair dye.
The completely protonated form of the dichromate ion is dichromic acid, H2Cr2O7 and can also be seen as the result of adding chromium trioxide to molecular chromic acid. When reacting with an aldehyde or ketone, dichromic acid exactly the same way. In organic chemistry, the chromic acid solution can oxidize primary alcohols to aldehyde and secondary alcohol to a ketone. but the tertiary alcohols and ketones are unaffected . During oxidation, the colour of chromic acid changes from orange to brownish green.
And that’s where the trouble begins. Choosing a source of chromium to make H2CrO4 from is a lot like choosing a favorite brand of bottled water. Beyond the packaging, they’re pretty much all the same. Depending on which textbook or instructor you have, however, you might see several different ways to do this, and it can be very confusing.
The key point is that Na2CrO4 (sodium chromate), Na2Cr2O7 (sodium dichromate), K2CrO4 (potassium chromate), K2Cr2O7 (potassium dichromate), and CrO3 (chromium trioxide) are all alike in one crucial manner: when they are combined with aqueous acid, each of them forms H2CrO4, and ultimately it’s H2CrO4 which does the important chemistry. Unfortunately I rarely see this point explained in textbooks. I remember this causing some confusion for me when I took the course. The K or Na ions present are just spectators.
H2CrO4 As A Reagent For The Oxidation Of Alcohols
Once H2CrO4 is formed, its reactions are pretty straightforward: it converts primary alcohols (and aldehydes) to carboxylic acids and secondary alcohols to ketones.
I wish I could tell you that navigating this confusion is ultimately rewarding due to the vast usefulness of H2CrO4 as a reagent. In fact, due to its high toxicity, chromic acid tends to find very little use in the organic chemistry laboratory outside of undergrad labs. There are far more useful reagents out there for performing these transformations.
Molecular chromic acid – H2CrO4 is similar to sulphuric acid (H2SO4) as both are strong acids, however, only the first proton is lost easily.
Dichromic acid – H2Cr2O7 is the fully protonated form of the dichromate (Cr2O7–) ion. Also, it is seen as the product of adding chromium trioxide (CrO3) to molecular chromic acid.
Uses of Chromic acid (H2CrO4)
Chromic acid acts as an intermediate in chromium plating.
It is used in ceramic glazes and coloured glass.
Chromosulfuric acid or sulfochromic mixture is a strong oxidizing agent that is used to clean laboratory glassware.
It has the ability to brighten raw brass and therefore it is used in the instrument repair industry.
In the year 1940, it was used in hair dye.
The completely protonated form of the dichromate ion is dichromic acid, H2Cr2O7 and can also be seen as the result of adding chromium trioxide to molecular chromic acid. When reacting with an aldehyde or ketone, dichromic acid exactly the same way. In organic chemistry, the chromic acid solution can oxidize primary alcohols to aldehyde and secondary alcohol to a ketone. but the tertiary alcohols and ketones are unaffected . During oxidation, the colour of chromic acid changes from orange to brownish green.