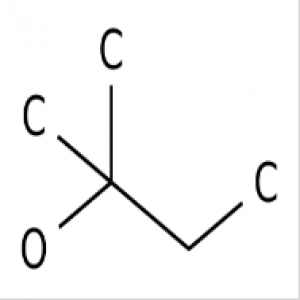
What is Ethyl Acetate boiling point?
Ethyl acetate boiling point is an organic ester compound with a molecular formula of C4H8O2. It is a colourless liquid with a fruity characteristic odour that is commonly recognised in glues and nail polish remover. Ethyl acetate boiling point is extremely flammable with a flashpoint of -4° C and a flammability rating of 3 and is also highly miscible with all common organic solvents (alcohols, ketones, glycols, esters) but only slightly miscibility in water. This product is commonly used as a solvent for cleaning, paint removal and coatings.
How is ethyl acetate boiling point made?
There are various methods for manufacturing ethyl acetate boiling point. Originally, it was synthesised by distilling ethanol and acetic acid in the presence of sulfuric acid. It is now primarily produced commercially via the Tishchenko method of condensing two equivalents of acetaldehyde using an alkoxide catalyst.
2 CH3CHO → CH3CO2CH2CH3
Another primary method is using Fischer esterification which involves reacting acetic acid with ethanol, a process accelerated by acid catalysis.
CH3CO2H + CH3CH2OH → CH3CO2CH2CH3 + H2O
Other methods include as a by-product of the oxidation of butane with acetic acid, the ethanolysis of polyvinyl acetate, and the alkylation of acetic acid.
A chemical stockist would have a bulk petrochemical storage facility to regulate this product. Storage is normally in a cool, dry and well ventilated facility away from oxidising agents. Ethyl acetate boiling point should be kept out of direct sunlight, heat and open flames. Ethyl acetate boiling point should be stored in drummed containers such as iso tanks made of stainless steel, aluminium or carbon steel.
A bulk solvent exporter would normally distribute this solvent in bulk vessels or tank trucks. For transportation purposes, ethyl acetate boiling point is classed as a flammable liquid with a fire hazard rating of 2. A full bulk chemical distributor would export the solvent throughout regions such as the UK, Europe, Africa and America. This product is a packing group 2.
What is ethyl acetate boiling point used for?
Ethyl acetate boiling point has many uses in the industrial and commercial industries as both a solvent and a diluent.
Ethyl acetate boiling point is used in various industrial applications such as in paints as a hardener, adhesives, paint and coating additives, degreasing solvents, active agents, processing aids and plasticisers. At a lower purity, it can be used in printing and pharmaceuticals. It is also used in coating formulations for wood furniture, agricultural, construction equipment, mining equipment and marine uses.
Laboratory uses include in mixtures used in column chromatography and extractions.
Ethyl acetate boiling point is an organic ester compound with a molecular formula of C4H8O2. It is a colourless liquid with a fruity characteristic odour that is commonly recognised in glues and nail polish remover. Ethyl acetate boiling point is extremely flammable with a flashpoint of -4° C and a flammability rating of 3 and is also highly miscible with all common organic solvents (alcohols, ketones, glycols, esters) but only slightly miscibility in water. This product is commonly used as a solvent for cleaning, paint removal and coatings.
How is ethyl acetate boiling point made?
There are various methods for manufacturing ethyl acetate boiling point. Originally, it was synthesised by distilling ethanol and acetic acid in the presence of sulfuric acid. It is now primarily produced commercially via the Tishchenko method of condensing two equivalents of acetaldehyde using an alkoxide catalyst.
2 CH3CHO → CH3CO2CH2CH3
Another primary method is using Fischer esterification which involves reacting acetic acid with ethanol, a process accelerated by acid catalysis.
CH3CO2H + CH3CH2OH → CH3CO2CH2CH3 + H2O
Other methods include as a by-product of the oxidation of butane with acetic acid, the ethanolysis of polyvinyl acetate, and the alkylation of acetic acid.
A chemical stockist would have a bulk petrochemical storage facility to regulate this product. Storage is normally in a cool, dry and well ventilated facility away from oxidising agents. Ethyl acetate boiling point should be kept out of direct sunlight, heat and open flames. Ethyl acetate boiling point should be stored in drummed containers such as iso tanks made of stainless steel, aluminium or carbon steel.
A bulk solvent exporter would normally distribute this solvent in bulk vessels or tank trucks. For transportation purposes, ethyl acetate boiling point is classed as a flammable liquid with a fire hazard rating of 2. A full bulk chemical distributor would export the solvent throughout regions such as the UK, Europe, Africa and America. This product is a packing group 2.
What is ethyl acetate boiling point used for?
Ethyl acetate boiling point has many uses in the industrial and commercial industries as both a solvent and a diluent.
Ethyl acetate boiling point is used in various industrial applications such as in paints as a hardener, adhesives, paint and coating additives, degreasing solvents, active agents, processing aids and plasticisers. At a lower purity, it can be used in printing and pharmaceuticals. It is also used in coating formulations for wood furniture, agricultural, construction equipment, mining equipment and marine uses.
Laboratory uses include in mixtures used in column chromatography and extractions.